Ethanol
Ethanol can be dehydrogenated to ethanal (acetaldehyde) over a catalyst (typically copper) at high temperatures. Depending on the catalyst, other products such as ethyl acetate can be formed.
CH3CH2OH ->[Cu] CH3CHO + H2
Methanol
Methanol can similarly be dehydrogenated to methanal (formaldehyde) over a catalyst (typically copper) at high temperatures.
CH3OH ->[Cu] CH2O + H2
Work Log
18 Oct 2019
Continuing with methanal. I made the copper impregnated carbon. Now I have to reduce it in-situ. I’m using aluminum + acid as my hydrogen generator. The acid is the semi-spent sulfuric and hydrochloric acid mix from the aluminum chloride synthesis. This mix is dripped slowly onto aluminum foil balls in a bit of water to retard the initial reaction.
I hope to get the hydrogen cell running soon so I can generate hydrogen on demand for cheap. Also, I have used just about every clamp and keck clip for this glass setup. I need some more…
For the initial run to decompose the copper nitrate (~170C), I vented the gasses directly out of the fume hood instead of leaving the bubbler in place. This is so I don’t have nitric acid contamination of the (already filled) formaldehyde collector. After the nitrogen dioxide evolution has ceased, I will reconnect the bubbler to visually determine the hydrogen flow rate.
As a side note, it is very difficult to measure the temperature of a 1cm diameter glass tube with an IR thermometer. You have to align the beam just right to get a measurement that doesn’t fluctuate wildly.
Well I’m stupid. I guess I didn’t let the catalyst cool long enough before starting the hydrogen… I had to clean up 100+ml of acid explosion. I’m going to try again tomorrow this time with a CO2 purge for good measure.
08 Oct 2019
Let’s make some methanal! (Shelepova et al. 2017)
- 20g activated carbon
- 1g copper as copper nitrate formed from 1.74g basic copper carbonate & nitric acid (5% w/w loading)
- dry
- reduce with hydrogen at 450C for 2 hours
13 Jul 2019
User jimmymajesty achieved excellent results by using air-oxidized copper plated nichrome wire in a ketene apparatus http://www.sciencemadness.org/talk/viewthread.php?tid=55&goto=search&pid=176566
19 Jun 2018
- Investigating the production of mesoporous carbon catalysts.
- Carbon has high selectivity compared to silica, but it suffers from short lifespan. Copper sintering seems the issue. Lack of interaction with the base material is the cause. Wang et al. (2018)
- Carbon can be deposited on silica supports with the layer of catalyst further deposited on that.
- Searching for a suitable mesoporous carbon precursor…
- Furfural alcohol might be ok since it can be made from agricultural byproducts and sulfuric acid followed by hydrogenation. (“FURFURAL” 1921)
- Sucrose could work Böhme, Einicke, and Klepel (2005) Ryoo, Joo, and Jun (1999)
- 52.5 kJ mol => 49 BTU; easily done with bunsen burner; heat loss is probably biggest problem
- chromite stabilization is another option but isn’t good for the environment
- instead of mesoporous carbon… graphene? Morales et al. (2016)
- I haven’t found anything on electrooxidation in DES
- As far as setup goes: something akin to Doug’s Lab Ketene Lamp but with a core of catalyst heated by resistance wire?
26 May 2018
Isolating various metallic catalyst precursors:
- Nickel (US 5 cent piece)
- available as nickel carbonate colorant: better to buy because of toxicity issues with electrolyzing nickel compounds
- Barium
- Gold (from previous experiments)
- Platinums
- probably best to buy it
Nickel
Hmmm… Extracting nickel from nickels:
Attempts using a polypropylene fabric bag to hold the nickels.
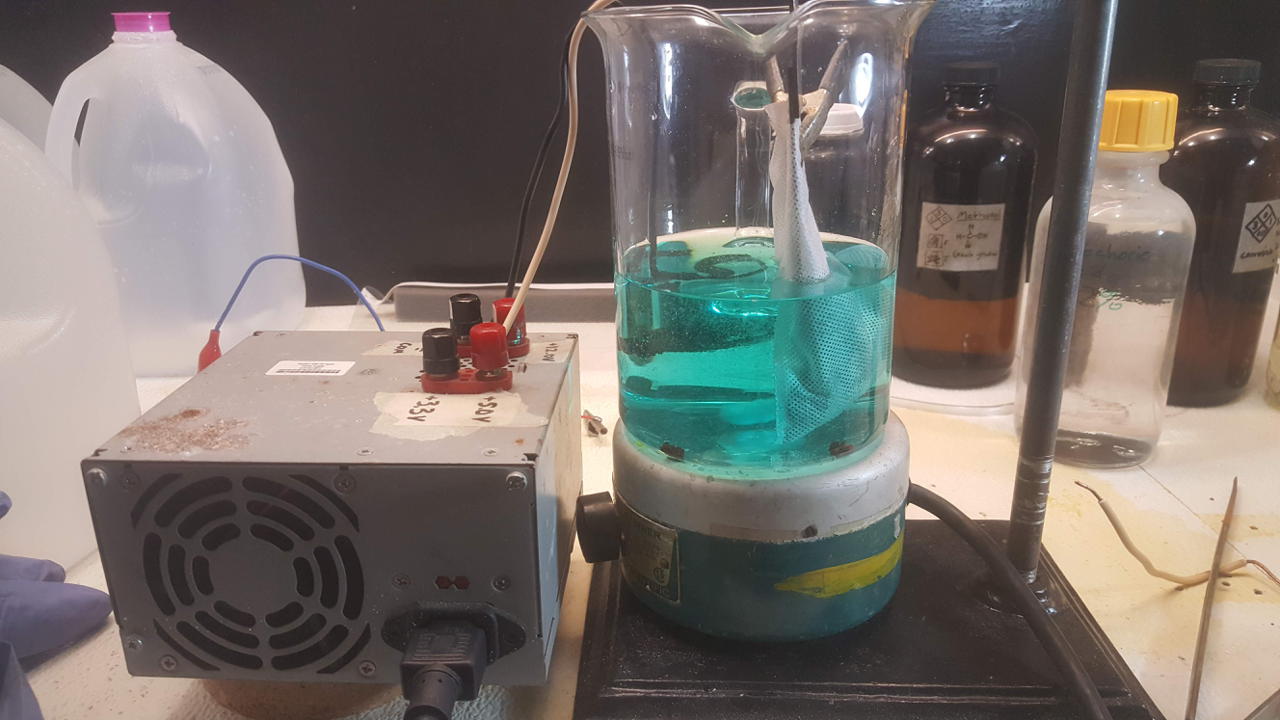

Final method that simply uses a basket made from copper wire. The copper plates out of solution while the nickel stays dissolved.

Barium
(rust converter) => cheaper as barium carbonate glaze

Bibliography
Böhme, Kerstin, Wolf-Dietrich Einicke, and Olaf Klepel. 2005. “Templated Synthesis of Mesoporous Carbon from Sucrosethe Way from the Silica Pore Filling to the Carbon Material.” Carbon 43 (9): 1918–25. https://doi.org/10.1016/j.carbon.2005.02.043.
Morales, M. V., E. Asedegbega-Nieto, B. Bachiller-Baeza, and A. Guerrero-Ruiz. 2016. “Bioethanol Dehydrogenation over Copper Supported on Functionalized Graphene Materials and a High Surface Area Graphite.” Carbon 102 (June): 426–36. https://doi.org/10.1016/j.carbon.2016.02.089.
Ryoo, Ryong, Sang Hoon Joo, and Shinae Jun. 1999. “Synthesis of Highly Ordered Carbon Molecular Sieves via Template-Mediated Structural Transformation.” The Journal of Physical Chemistry B 103 (37): 7743–6. https://doi.org/10.1021/jp991673a.
Shelepova, Ekaterina V., Aleksey A. Vedyagin, Ludmila Yu. Ilina, Alexander I. Nizovskii, and Pavel G. Tsyrulnikov. 2017. “Synthesis of Carbon-Supported Copper Catalyst and Its Catalytic Performance in Methanol Dehydrogenation.” Applied Surface Science 409 (July): 291–95. https://doi.org/10.1016/j.apsusc.2017.02.220.
Wang, Qing-Nan, Lei Shi, Wei Li, Wen-Cui Li, Rui Si, Ferdi Schüth, and An-Hui Lu. 2018. “Cu Supported on Thin Carbon Layer-Coated Porous SiO2 for Efficient Ethanol Dehydrogenation.” Catalysis Science & Technology 8 (2): 472–79. https://doi.org/10.1039/C7CY02057K.

















