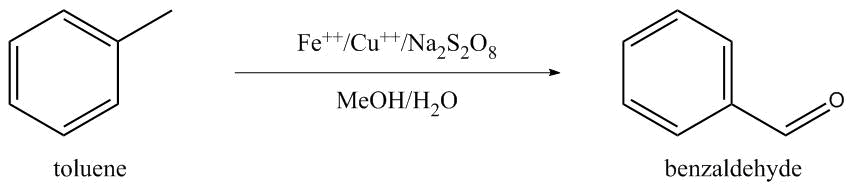Introduction
Benzaldehyde is a precursor to a huge number of organic compounds. Synthesis in a high yield at a low cost is the ultimate goal. Expensive reagents are to be avoided. Bonus points for doing it quickly.
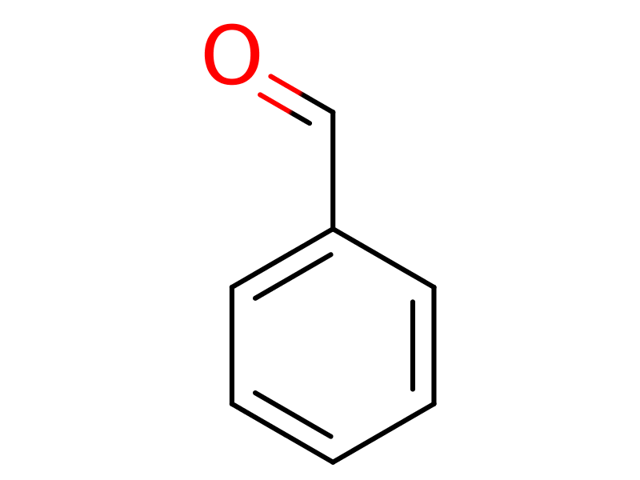
Properties
Methods
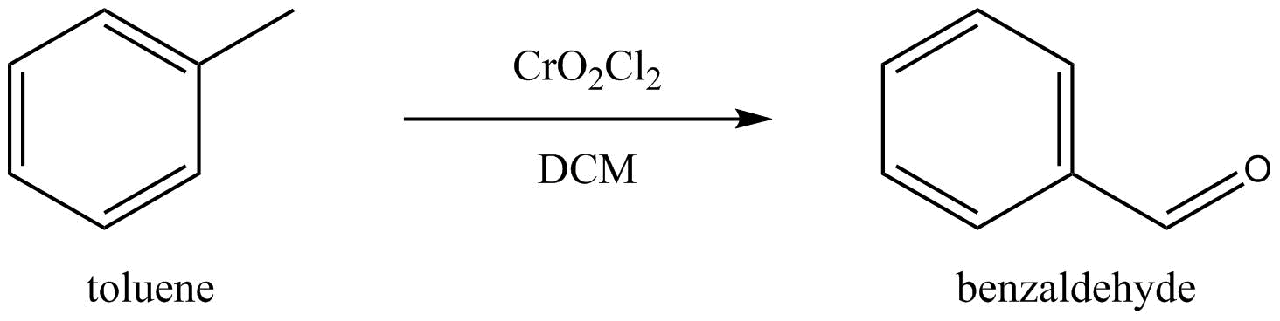
Chromyl Chloride and Dichloromethane
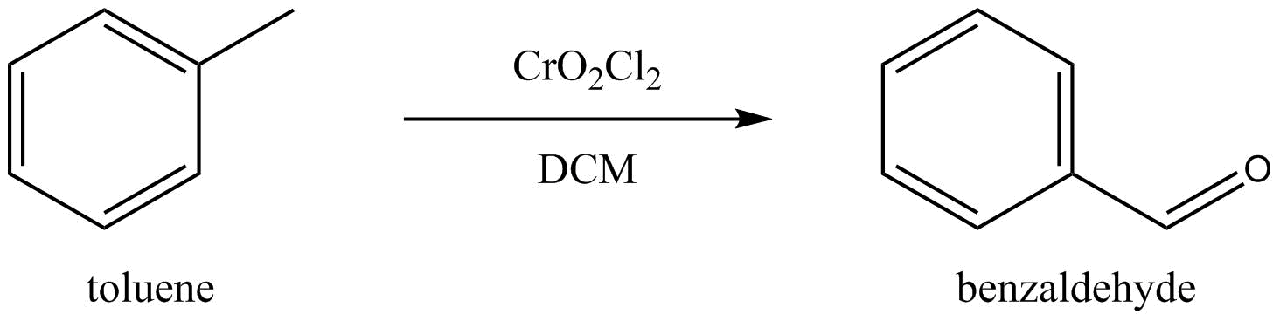
Attempt by Dennis Phillips aka NileRed: 51% yield (Dennis Phillips 2017)
Benzoic Acid and Hypochlorite
- 12% yield based on Benzoic Acid. (Scott Daniel 2017)
Sodium Persulfate and Iron-Copper Catalysts
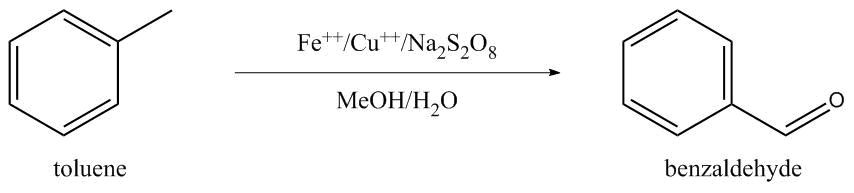
Ferrous-Copper Catalyst: Toluene (7.6 g.), water (35 ml.), ferrous sulphate (0.110 g.) heptahydrate, cupric acetate (0.072 g.) and methanol (8 ml.) are placed in a 250 ml. reactor.
Sodium persulphate (47.05 g.) in an aqueous-methanol solution of sodium persulphate is added slowly to the mixture which is maintained at 70° C., in an atmosphere of nitrogen and under agitation.
The organic phase is separated after two hours and the aqueous phase is extracted with ethyl ether.
The combined organic phases are distilled to afford 8.29 g. (95% yield) of very pure benzaldehyde (compared against a pure sample). (Maggioni 1979)
Attempt by sum_lab gave single-digit yields. (sum_lab 2018)
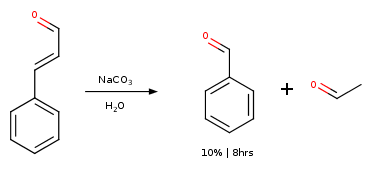
Cinnamon Oil
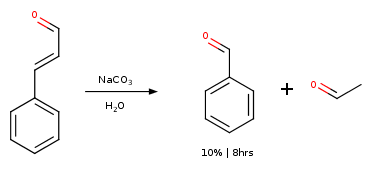
Attempt by NileRed yielded 10% on weight basis of cinnamon oil. (NileRed 2016)
Pyrolysis
Work Log
19 Apr 2020
Attempting benzaldehyde by peroxide oxidation of toluene in Ethaline.
I don’t have any ferric chloride, so I followed the [Make Ferric Chloride | NurdRage] video procedure.
- 20 g scrap iron
- 100ml water
- 100ml 12M HCl
- top up to 200ml with HCl
- 200ml 3% H2O2
13 Jul 2018
- started research on various methods used in the literature
- fiddled with Marvin for 5 hours: Chemdraw is wayyyy better
Bibliography
Dennis Phillips. 2017. “Making Benzaldehyde (Étard Reaction).” https://www.youtube.com/watch?v=L8kFJPEZdwM.
Maggioni, Paolo. 1979. Process for preparing aromatic aldehydes and ketones. US4146582A, issued March 1979. https://patents.google.com/patent/US4146582A/en?oq=4%2c146%2c582.
NileRed. 2016. “Making Benzaldehyde (from Cinnamon Oil).” https://www.youtube.com/watch?v=Fui19FPh0R0.
Scott Daniel. 2017. “Benzaldehyde via Hypochlorite Oxidation of Benzyl Alcohol.” https://www.youtube.com/watch?v=ExogvX4vD78.
sum_lab. 2018. “Benzaldehyde Attempt 1.” https://www.youtube.com/watch?v=G_PSJAtfmso.