Growth Considerations
- total N
- NO3/NH4 ratio
- salinity
- root zone O2
- temperature (root/shoot)
Many medicinal plants have a tendency to bioaccumulate heavy metals. This is a big problem for traditionally grown plants especially when the fertilizer is organic in nature. (Nookabkaew et al. 2016) Similarly, anthroponegic sources of cadmium are often the culprit of contamination (e.g. biosolids or industrial runoff).
Synthetic fertilizers are not immune to this contamination, however. Cadmium naturally occurs in phosphate rock. Synthetic phosphorus fertilizers are directly produced from this rock and therefore have higher than background concentrations of cadmium.
Melissa officinalis
I routinely use lemon balm in herbal tea mixtures. My usual mix is 50:50 with lavender flowers. I just like the lemony scent.
Lemon balm has been grown successfully in soilless media. (???)
There appears to be some benefit to water deprivation for essential oil concentration but has variable affects on total yield. (H et al. 2010) (Radácsi et al. 2016)
Lemon balm grows in shade and full sun.
Centella asiatica
Gotu Kola is purportedly used for cognitive enhancement and enhanced wound healing.
Triterpenoids seem to be the most important compounds in gotu kola. (Moyano et al. 2009) These are carbohydrate secondary metabolites and have reduced production when the total nitrogen exceeds 60 mM in shoot culture. Similarly, carbohydrate supplementation increased asiaticoside production while copper inhibits it. (Prasad, Pragadheesh, et al. 2012)
High yields of pharmaceutical quality gotu kola are seen with hydroponics. (Prasad, Mathur, et al. 2012)
Gotu Kola has been used for phytoremediation of waste water. (Nuwansi et al. 2019)
Plant genetics are important for production.
Piper auritum
Grows in tropical climates. Potential for hydroponic cultivation. Will grow several meters tall with ~30cm wide leaves. Propagates by rhizomes, seed.
Possible source of safrole (2-4% essential oil in leaves; 87-94% safrole).
P. auritum could be grown as undergrowth in a larger timber plantation.

P. auritum has a spider mite problem. So I gave it a shower.

Bacopa monnieri
Withania somnifera
Curcuma domestica
Sceletium tortuosum
Kanna is an African succulent that does not do well with overwatering. It thrives in high light and high heat.
Kanna may drop all of its leaves during the summer and regrow them in the winter.
Kanna may be suitable for a fertigation based hydroponic cultivation.
It was observed that more optimal vegetative growth was not necessarily desirable in terms of alkaloid concentration, which suggests that reasonable amounts of stress could increase alkaloid concentrations in the plant. Chapter 3 found soilless media with higher water holding capacity (media containing coco-peat and vermiculite) yielded more desirable results in terms of vegetative growth than media that did not (media containing pure silica sand and/or perlite). Too frequent (every week) or infrequent (every fifth week) fertigation is not ideal for vegetative growth, instead plants reacted significantly to fertigation at 3 week intervals. One can therefore suggest that well drained, yet high water holding capacity soilless media in conjunction with mid-frequent fertigation intervals would yield the best results in terms of vegetative growth.
(Faber 2019)
Preparation method seems to be important. Perhaps to reduce oxalate content or even to enhance psychoactivity.
When the subjects further attempted to ingest unfermented plant material which had been freeze-dried, by mouth, the acidity was most objectionable and the exercise was discontinued. …perhaps the physical crushing of the plant and the fermentation process may, in some way, ameliorate the potentially harmful effects of oxalic acid.
The fermentation method involves placing the whole plant in a sealed plastic bag in the sun for several days, opened and mixed, then sealed for a total of eight days. The plant material is then dried in the sun in a thin layer.
Kanna is also traditionally prepared by burying the whole plant in hot sand left under a fire pit for one hour or overnight. (Smith et al. 1996)
Nicotiana tabacum

Nicotiana rustica
Previous tests have shown that N. rustica is well suited for Kratky style hydroponics.



Hypericum perforatum


Rhodiola rosea
Calendula officinalis


Phalaris aquatica
Seeds are direct sowed in coconut coir.
Ephedra californica
Seeds are soaked in 3% hydrogen peroxide for 10 minutes before sowing in peat pellet and rockwool.
Borago officinalis

Cantharanthus roseus
Germination is a little difficult. I am using 10mM KNO3 solution to wet a rockwool grow cube. Two seeds are placed in the indentation and covered with a bit of rockwool to ensure that the seeds aren’t exposed to light. Two seeds were also placed in traditional peat pellets and covered as well.
Ipomoea tricolor
Seeds are soaked in 3% hydrogen peroxide for 10 minutes before sowing in peat pellet and rockwool.
Dysphania ambrosioides
Lavandula vera
Crassula ovata ‘Ogre Ears’
Sedum
Senecio

Huernia
Aeonium
Epiphyllum
Theobroma cacao
I received 21 ICS95 cacao cuttings from Puerto Rico. They were immediately dipped in IBA rooting hormone, planted in a 50:50 vermiculite:coconut coir potting media, and watered with half strength (masterblend) nutrient solution.
Several days later I pulled 9 cuttings from the pot and placed them in my ultrasonic mist bench. The others were repotted in the same verm:coir mix in a smaller container. I attempted to use a plastic bag as a humidity dome, but it became too unwieldy. The mist bench has an inverted clear plastic tote which fits perfectly.
Thus far I have seen little to indicate they are growing roots. Apparently mist benches have the tendency to osmotically pull nutrients from the cuttings when used with pure water (which I did).
Yep… rot :(

Curio radicans
Wasabia japonica
Tradescantia pallida


Appendix A Stock Solutions
NaOH 1N
- 20.00g sodium hydroxide
- bring to 500g total with distilled H2O
BAP 1mg/ml
- 100mg BAP
- 2-5ml 1N NaOH solution
- to 100ml total volume with H2O
Kinetin 1mg/ml
- 100mg kinetin
- 2-5ml 1N NaOH solution
- to 100ml total volume with H2O
IAA 1mg/ml
- 100mg IAA
- 2-5ml 1N NaOH solution
- to 100ml total volume with H2O
L-Arginine 10mg/ml
- 250mg L-arginine
- to 25ml H2O
Citric Acid 10mg/ml
- 250mg ascorbic acid
- to 25ml H2O
Ascorbic Acid 10mg/ml
- 250mg ascorbic acid
- to 25ml H2O
Antioxidant Soak Solution
- 250mg ascorbic acid (0.1%)
- 250mg citric acid (0.1%)
- to 250ml H2O
Work Log
TODO
- [ ] research antifungal treatments
- [ ] research OTC antibacterial soaps
09 Oct 2021
I ate a kratom leaf. It was very bitter and obviously active.
The kratom desperately need a new home or a serious trim. Many branches are curving because of the walls of my closet.
25 Sep 2021
- establishment of tissue culture
- seed germination
- vegetative propagation
- artificial seed germination
- root culture
- callus culture
- response to growth conditions: heat, photoperiod, light quality, light quantity
- response to hydroponic cultivation
- active ingredient identification
- active ingredient phytochemistry
- active ingredient enhancement
- sexual reproduction and hybridization
- seed quality in response to environmental/heritable factors
- heritability of phenotypes
- genetic lineage
- biomarkers/phenotypic-markers of active ingredient content
- harvest protocols
- post-harvest protocols
22 Sep 2021
Lots of work on the plant database.
13 Sep 2021
Ammonium nitrate can also be made by combining calcium (ammonium) nitrate with ammonium carbonate.
11 Sep 2021
No real activity from the kratom cuttings. Not that there should be, but I’m impatient. One might have bacterial contamination but I can’t tell if it is just leftover minerals from the sterile tap water rinse I used instead of distilled water.
08 Sep 2021
Imidacloprid is also an option. Looking at this product label it seems that 3.7mg imidacloprid per square foot diluted in 100ml is appropriate.
06 Sep 2021
PLANT TISSUE CULTURE CSIR | YouTube
I finally got up the courage to try the first kratom multiplications. I selected 1 cutting from each of Thai, Indo, Malay, Bumblebee plants. I cut at the midline between the second and third nodes from branches off the main trunk.

I then made cuts at the midline between the first and second nodes.

This gives two stem cuttings: first (distal) node and second (proximal) node. The second node has all of the leaves removed and is used for micropropagation. The first node has the leaves trimmed and is used for traditional propagation. Both are submerged in tap water to the next step.
All nodal segments are then submerged mild detergent solution (1 drop dish detergent per 200ml tap water) with a splash of hydrogen peroxide (~0.1% solution) for 30 minutes. Every few minutes they are swirled to mix.

Segments are then rinsed with tap water. I’ve had problems with fungal contamination in the past. This is the point where I should soak in an antifungal. I’ll also look into OTC antibacterial soaps.
Micropropagation segments (MSB0003, MST0003, MSI0003, MSM0003) are transferred to sterile containers in flow hood. Soaked in 70% EtOH solution for 30 seconds. Soaked in 0.56% sodium hypochlorite solution (diluted bleach with a few drops of tween-20 per liter) for 25 minutes. Rinsed twice in sterile water. Sown in MS0002.

Traditional propagation segments (MSB0004, MST0004, MSI0004, MSM0004) were dipped in store-bought IBA-based rooting hormone and planted in rockwool cubes. Quarter strength masterblend hydroponic media (MB002) was added to the shot-glasses which held the cubes in place. The four segments were placed inside HDPE bag in indirect 16-hour light.

Both are incubated in 16-hour light cycle with the rest of my plants.

One of the tygon tubing sections fell off the syringe filters in the MST0003 jar. It’s now embedded in the agar. This shouldn’t be a problem as long as the filter stays adhered.
Aaaannnnddd I forgot to soak the explants in antioxidant solution before sowing. The media contains antioxidants, so hopefully they will survive.
I also completely botched the sterilization procedure. I followed my usual seed sterilizing procedure. I think it is likely that these explants will die because of the long soak times in bleach. Ahhh well. It was a good first take.
A bunch of extra nodes were clipped from the main cuttings during collection. I didn’t think I would root these, so they were mixed with the removed leaves. But I ended up not wanting to waste them. I dipped them in hormone and put them in a beaker with a bit of water and covered it with plastic wrap.

I also put them straight into the incubator instead of the 48-hour darkness incubation period as prescribed.
05 Sep 2021
Stem or branch segments 2-3 cm long can be surface sterilized in 70% ethanol for 1 min followed by 15 min in 7% calcium hypochlorite containing 0.1% Tween 20. Shoot tips do not require ethanol treatment and only 10 min of calcium hypochlorite.(Ahuja 1993)
Explants were placed under running tap water with detergent for 30 min to remove any foreign contaminants.
After washing, explants were dissected and surface sterilized in a laminar air flow hood with rinsed with ethanol 70% for 2-3 min then were washed with distilled water three times for 2-3 min after that.
It is recommended for this study to be used among the different sterilization variants, sodium hypochlorite was the most effective treatment with 50% survival rate at V5 (15% for 5 min) and 60% survival rate at V4 (10% for 10 min).(Ghasheem, Peticil, and Venat 2018)
It can be really confusing when a paper says “5% bleach” when they really mean 1:20 diluted 5.25% sodium hypochlorite in water, so actually 0.26% sodium hypochlorite.
04 Sep 2021
Bought a really old 0.1mg analytical balance. The box was trashed in shipping.

The top shield glass was definitely broken in shipping.

I don’t know if the tens place led was damaged in shipping or not, since the listing never showed it.

In any case, I was still able to make some stock solutions.

- 25.6mg kinetin
to 25ml distilled water
- 25.8mg IAA
to 25ml distilled water
- 25.7mg BAP
to 25ml distilled water
- 250.2mg citric acid
to 25ml distilled water
- 250.5mg ascorbic acid
to 25ml distilled water
For 750ml M. speciosa shooting media (id: MS002)
- full strength MS media (4.2g premade media + MES)
- 22.5g sucrose
- 6g agar
- 3.75ml ascorbic acid stock solution (37.5mg)
- 1.875ml citric acid stock solution (18.75mg)
- 75mg activated charcoal (300 mesh)
- 0.375ml 6-BAP stock solution (0.375mg)
- 0.188ml kinetin stock solution (0.188mg)
- 0.075ml IAA stock solution (0.075mg)
Heated mix to 90C then dispensed 150ml into 5 1qt large mouth jars. The jar lids have 2.5cm teflon syringe filters secured with silicone as described previously. Sterilized at 115psi for 10 minutes.

Sprayed the kratom down with acetamiprid.

11 Aug 2021
Kratom is doing well.




The fungi controller is up and running.
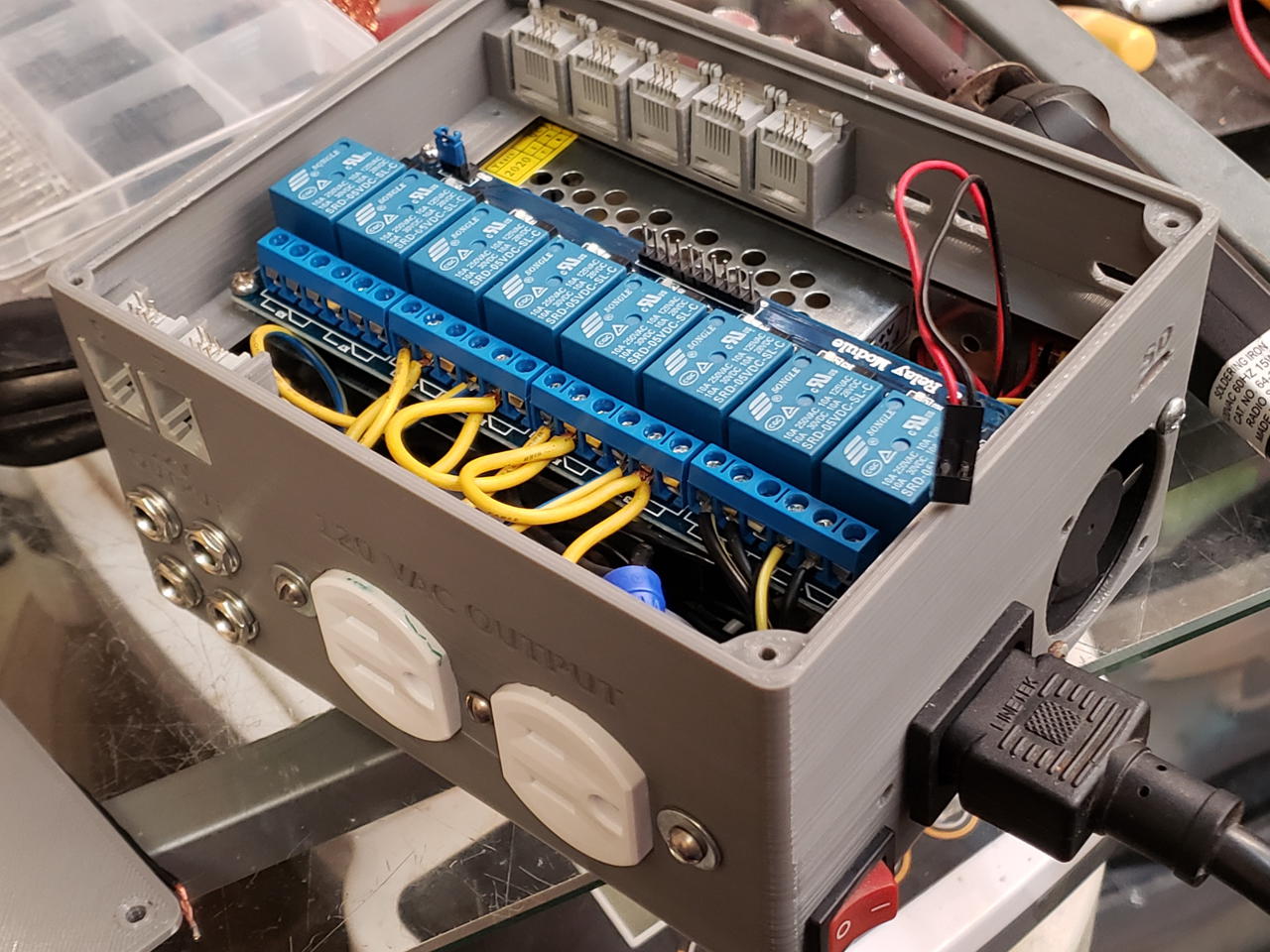
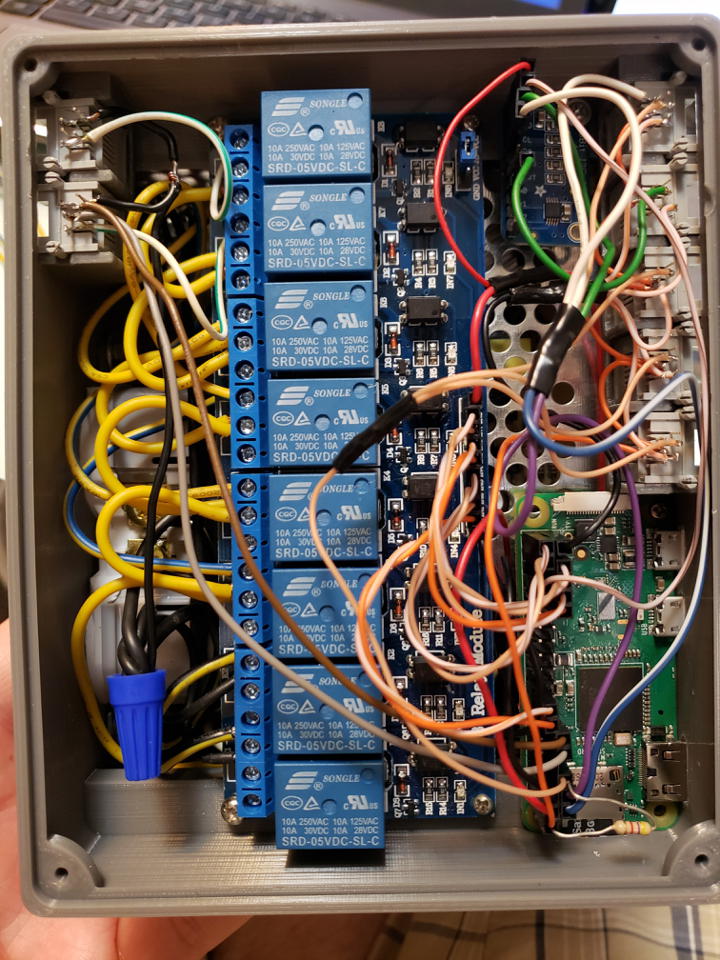
I had to make a few modifications. For example, the SD card slot needs to be wider to allow removal. Some edges need to be chamfered. Mounting holes should be added.
06 Aug 2021
The foam filter test is terminated. The 90 degree bend in the updraft tube combined the fine bubbles reducing the aerial spray. This might not be a problem for established plants, but the foam filter plant had visibly reduced root growth. No other problems were noted.
Aeration using foam filter

Aeration using traditional sparger 
04 Aug 2021
Beautiful crystals of ferrous sulfate heptahydrate formed. I decanted the majority of the liquid and vacuum filtered the rest. Rinsed the crystals with 95% EtOH. Dried the crystals at <50C overnight. Recovered 148g.
The remaining liquid was reduced by about 40% and put in the refrigerator for a second crop of crystals. These were added back to the fertilizer grade bag. The liquid was discarded.
The insoluble material left behind in the filter.

The, now, much clearer solution.

The resultant crystals.

Drying the crystals in a dehydrator.

The kratom trees are doing well.

Roots systems are developing nicely. I had to add about 1L of nutrient solution to each of the buckets yesterday. I will definitely need a dosing system in the near future.
01 August

04 August

The damned mealybugs have persisted despite my continuous checking and spraying. Fuck those things.
03 Aug 2021
Ferrous sulfate did not precipitate overnight. I set the flask out on my desk to warm up to room temperature. With a few shakes, crystals began to form. I put the flask back in the refridgerator overnight.
Made some gas exchange lids for 1 quart mason jars. Just some 25mm PTFE 0.22um syringe filters glued to the lid using RTV silicone. I used a short piece of tygon tubing to secure the internal connection.


02 Aug 2021
Purified some ferrous sulfate heptahydrate today.
- 1L tap water
- 22ml concentrated sulfuric acid (18M hardware-store drain opener)
- makes ~1L 0.4M H2SO4 solution
- 500g total Hi-Yield Iron Sulfate; in portions
- heated to boiling
- filtered
- crystallized in the refridgerator (2-5C)
Do not crystallize below 0C.
26 Jul 2021
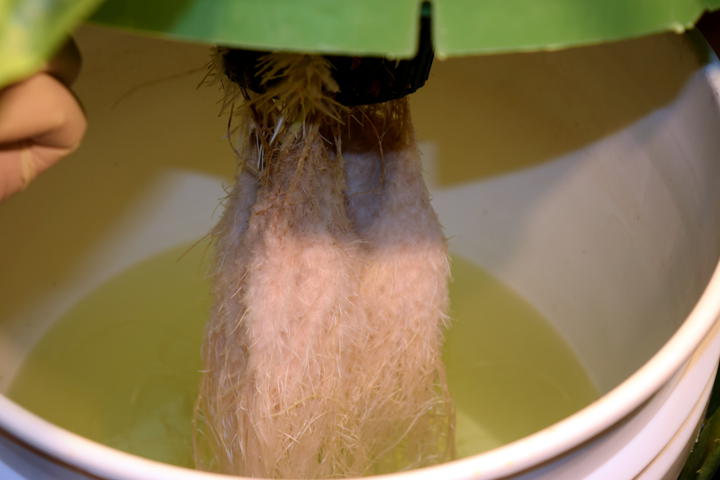
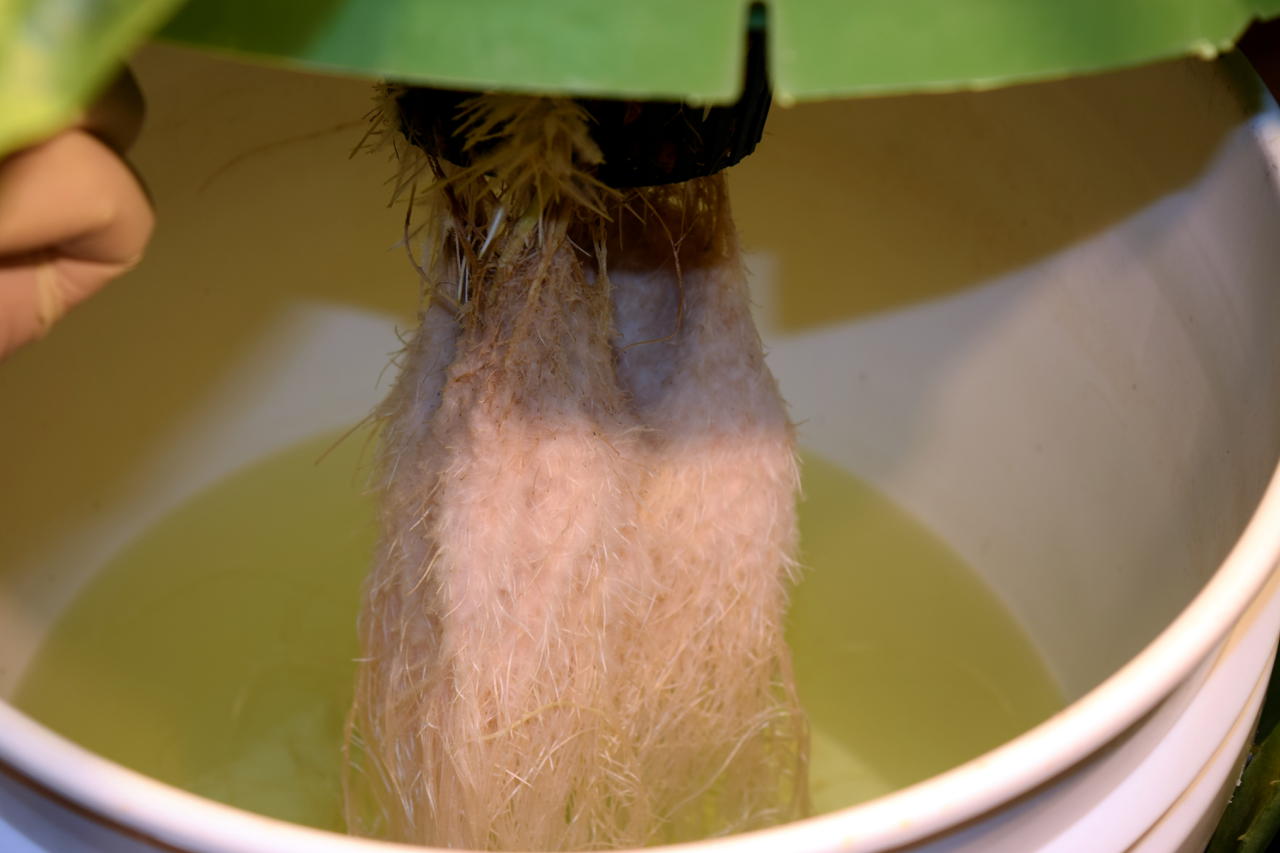
The M. speciosa plants are doing well after their transplant to hydroponics. I tested the bumblebee cultivar first since I have a spare. Just a simple DWC using half-strength general purpose “Masterblend” nutrients. After 24 hours with no wilting or other deleterious effects, I did the other three plants in a similar manner.
The roots are rinsed to remove as much soil matter as possible.
Initial test with bumblebee.
Completed set up with all four plants.
Some of the root balls wouldn’t quite fit into 6" net pots.
I decided to test a sponge filter in one of the buckets. The rest have 0.5 micron stainless steel spargers. MSI001 has the sponge filter.
The volume of air going through the sponge filter is less than ideal. The unit is designed for high flow and low pressure. I would like to design one for 3D printing that has better aeration with finer bubbles.
I think it is time to start generating shoots.
Nodal explants harvested during spring season from the lopped tree differentiated the maximum number of axillary shoots (5.3 ± 0.82 per node) on full-strength Murashige and Skoog (MS) medium containing 3.0 mg L−1 6-benzylaminopurine (BAP) and additives (25 mg L−1 each of adenine sulfate, L-arginine, and citric acid and 50 mg L−1 ascorbic acid).
The greatest number of shoots (13.4 ± 1.26) with an average length of 6.2 ± 1.03 cm was produced after 4 wk on MS medium containing 0.5 mg L−1 BAP, 0.25 mg L−1 kinetin (Kin), 0.1 mg L−1 Indole-3-acetic acid (IAA), additives, 100 mg L−1 activated charcoal (AC), and 0.8% (w/v) agar.(Patel, Lodha, and Shekhawat 2020)
So I need stock solutions of citric acid, ascorbic acid, kinetin, IAA, and BAP. I also need to grind some activated carbon. I don’t have any arginine or adenine sulfate right now. Neither seems to be especially necessary, so I will try without. Arginine is easy enough to get, but adenine is insanely expensive. I will also need 1N NaOH.
That particular paper doesn’t use filtering for the PGRs. Everything is added prior to autoclaving.
Growth regulators | Sigma Aldrich
I have a bunch of 25ml volumetric flasks. That would require the measurement of approximately 25mg of PGR. That is going to be difficult without a lab scale…
18 Jul 2021
The fungus gnats are getting annoying. The pasteurized soil that the kratom plants are in seems to have a high potential for mold. This doesn’t hurt the plants, but it attracts fungus gnats.
So I think I want to replace the soil with hydroponics. There are a few examples scattered across the internet of people using DWC hydroponics with kratom.
It seems that kratom can tolerate as much fertilizer as you can give it.(Zhang et al. 2020)
Of my own plants, they are also quite tolerant of water logging.
Instead of obtaining verified accessions, why not donate some? SERNEC
17 Jul 2021
I received the luer-lok bulkheads today. They are perfect. Now I just need the boxes.
16 Jul 2021
I need to make some of the MS media ingredients soon as I am about out of the premix I bought. I also need to find/make some ammonium nitrate. My usual source has since switched to calcium ammonium nitrate. I have nitric acid and ammonia, so I can make it in a direct, albeit costly, manner if I need to.
Ferrous sulfate heptahydrate:
Crystallise the sulfate from 0.4M H2SO4, or precipitate it from an aqueous solution with EtOH. It is efflorescent in dry air, and is converted to the tetrahydrate at 57o, then to the monohydrate (white-yellow) at 65o [or by heating the heptahydrate (blue-green crystals) in a vacuum at 140o; the anhydrous salt forms white crystals]. The solubility in H2O is 0.45g/ml(77C) and 0.36g/ml(90C) for the monohydrate, 0.66g/ml(0C), 0.21g/ml(10C), 0.29g/ml(25C), 0.40g/ml(40C) and 0.51g/ml(54C) for the heptahydrate; (Armarego 2017)
Manganouse sulfate monohydrate:
Crystallise it from water (0.9ml/g) at 54-55C by evaporating about two-thirds of the water. It dehydrates above 400C.
06 Jul 2021
Seeds!
70% ethanol solution prepared from “Everclear 190 proof alcohol”. 500ml 95% ethanol mixed with 178ml distilled water. Previously made (~200ml) batch added to larger container.
0.6% sodium hypochlorite solution made by mixing 64ml 7.5% commercial bleach with 736ml water (to 800ml) plus three drops tween 20.
Built manual dispensers for both solutions. Just a simple switch and cheap peristaltic pump.



Also built a glass bead sterilizer. I used an old 50ml ceramic flask heater with some silica gel beads and a PID temperature controller.
The whole setup:

It works fine but the dispensers should be stationary and be motion triggered.f
03 Jul 2021
2016 - Steve Arthur (Micropropagation) 6th Annual Cattleya Symposium
02 Jul 2021
Meristem culture procedure | YouTube
M. speciosa
Mother explants can be detached from their shoots and repeatedly multiplied. Alternatively, daughter shoots can be repeatedly subcultured. (Patel, Lodha, and Shekhawat 2020)
The original paper calls for mercuric chloride sterilization. Ya… not doing that.
Exudates are a problem:
The problem of phenolic exudation/leaching was observed during culture initiation. To conquer this, sterilized nodal explants were treated with the sterile pre-chilled antioxidant solution (ascorbic acid and citric acid 0.1% (w/v)) for 18 to 20 min prior to inoculation.
Additionally, such initial exposure of explants to dark/diffused light conditions for a certain period can promote morphogenesis by axillary meristem activation as reported by Panwar et al. (2018).
Take explants in the spring:
Explants collected during the spring season were better in terms of percentage bud-breaking response, early bud-breaking, or days to respond and showed the least contamination in this study.
Cytokinins are necessary.
Explants cultured on MS basal medium (control) did not show any bud-breaking.
Arginine for shoot elongation
According to Greenwell and Ruter (2018), the molecular structure of arginine (C6H14N4O2) includes two extra nitrogen (imino) groups, and this higher percentage of reduced nitrogen (32.2%) may have been responsible for the improved shoot elongation (3.69 ± 0.59 cm).
Adenine sulfate can improve shoot induction and health. It’s addition was not tested and, considering the price, I am not sure if it is worth it.
In additives, adenine sulfate may have complemented the effect of cytokinins owing to its base structural similarity to cytokinins (Chhajer and Kalia 2017).
Subculturing seems to work indefinitely.
The shoot cultures were maintained for 2-y by regular subculturing every fourth/fifth week without any change in growth and vigor.
Rooting with 500mg/L IBA
The basal end of the shoots treated with 500 mg L−1 IBA for 5 min proved the best in which 90.1% of shoots formed the highest number of roots (8.5 ± 0.97 per shoot) with an average length of 9.40 ± 1.06 cm, after 5 wk (Fig. 2e).
The best results from NAA alone achieved only 66% rooting. The two were not tested together.
Afterwards, successfully hardened plants with more than 80% transplant survival rate were transferred to the nursery and subsequently to the field.
The micropropagated plants exhibited normal morphology and growth characteristics that are phenotypically analogous to the mother plant.
Sterilization protocol from (Mohamad Zuldin et al. 2013).
The explants were washed thoroughly with running tap water for 30 min. The explants were then sterilized with 70% alcohol for 15 seconds and were washed three times. The explants were soaked for 30 min in 40% clorox bleach that contained three drops of Tween-20 and were then washed three times with distilled water. Next, the explants were dried on petri plates that contained a layer of filter paper. After trimming the cut size, the surface-sterilized explants were planted on the culture medium.
Micropropagation Protocol
- cut one-node segments (4-5cm) of new growth from mother plant
- surface sterilize
- ?
- soak in sterile, antioxidant solution for 20 min (0.1% w/v ascorbic and citric acid)
- inoculate on sterile shoot multiplication media
- full strength MS
- 0.8% agar
- 3% sucrose
- 0.5mg/L BAP
- 0.25mg/L kinetin
- 0.1mg/L IAA
- 25mg/L L-arginine
- 25mg/L citric acid
- 50mg/L ascorbic acid
- 100mg/L activated charcoal
- 25mg/L adenine sulfate (not added)
- incubate in darkness 2 days
- incubate at 26C; 16hr photoperiod; 40-50 umol/m2/s PFD; 60% RH;
- shoot buds in 1-2 weeks with full cycle taking ~4 weeks
Rooting Protocol
- excise individual shoots from multiplied cultures
- dip base in 500mg/L IBA for 5 minutes
- inoculate onto sterile soilless growing media
- peat
- verm
- perlite
- 1/4 strength MS macro salts
- incubate in greenhouse conditions (28C; 85% RH)
- after root initiation, slowly acclimate to low RH and high temp
- gradually remove caps over 2-3 weeks
After complete removal of caps, plants can be transferred to potting media as usual.
01 Jul 2021
Starting new seeds. Full strength MS + 1g/L MES + 8g/l agar + 20 g/L sucrose.
- 750 ml mix
- 4.13g MS powder premix
- 6g agar
- 15g sucrose
07 Jun 2021
Moved the fungi controller to Mycodo.
01 Jun 2021
The research [20] provides clear evidence that, for the embryo-to-plantlet development under photoautotrophic conditions, the use of Magenta vessels and modified RITA-bioreactor is less effective at promoting shoot and root growth both in and ex vitro compared with the TRI-bioreactor. (Gupta and Ibaraki 2006)
RITA and Magenta boxes work for plants that tolerate complete and total immersion in the growing media. For rooting, you only want a portion of the plant submerged. That’s where the TRI-bioreactor (Temporary Root Immersion) excels.
The plantlets are put into individual receptacles with an inert support medium (vermiculite and paper waste in this instance). This medium can be directly transferred to final potting soil without disturbing the roots. Autotrophic media results in better survival.
The advantages of growing plants in an environment with reduced relative humidity are manifold such as development of functional stomata, increased wax deposition all of which can, in turn, prevent water loss when transferred ex vitro and thus increase the chance of survival and subsequent growth. Furthermore, in TRI-bioreactor the environmental parameters are maintained in such a way that the difference between the in and ex vitro conditions is minimum, as a consequence when the plants are transferred ex vitro they are capable to photosynthesize normally and thus can easily overcome the transition stress during the first week of ex vitro condition.
The original paper used somatic embryos of Coffea arabusta.
Nodal cuttings of coffee plantlets (Coffea arabusta) were cultured in Magenta vessels containing hormone‐free MS (Murashige and Skoog, 1962) medium supplemented with 20 g l–1 sucrose. After 4 weeks of culture, regenerated leaves were collected and cut into pieces (10 × 5 mm) and were placed in a Magenta vessel containing modified MS medium (for details, see Afreen et al., 2002). Agar (8 g l–1; Kanto Chemical Co., Tokyo, Japan) was used as a gelling agent and 30 g l–1 sucrose was added in the medium.
Cultures were placed in a growth chamber with an air temperature of 23 °C and a 16 h d–1 photoperiod provided by cool‐white fluorescent lamps (National Co., Tokyo, Japan). The ambient CO2 concentration was 400 µmol mol–1 and the photosynthetic photon flux (PPF) was 30 µmol m–2 s–1 measured on the empty culture shelf. Somatic embryos developed within 9–12 weeks of culture. Cotyledonary stage embryos were selected for use as experimental materials.(AFREEN, ZOBAYED, and KOZAI 2002)
26 May 2021
3 segment displays are only $2.26. Mini swirling agitators with weight activated timers would be cool. After injecting sterilant, the containers can be placed in one of the agitator receptacles. The time automatically starts.
I think a larger touchscreen panel would be better than individual displays. The cheapest 3.5" resistive touch screens are $16 each and HDMI versions around $25.
I can’t find ferric tartrate dihydrate despite it being a supposed ingredient in Hoagland’s No. 2 and Vacin/West media. It doesn’t exist on either the sigma website (which sells Hoagland No. 2 and lists ferric tartrate dihydrate as and ingredient) or on PubChem.
The original Hoagland and Arnon paper (the revised edition from 1950 anyway) calls it “iron tartrate” and never alludes to the chemical structure.(Hoagland and Arnon 1950) Vacin and West do indeed spell out the formula as the dihydrate.(Vacin and Went 1949)
This also leads me to question some of the other odd ingredients in Sigma’s collection. Most of them list “MgSO4” but anhydrous magnesium sulphate is uncommon. The heptahydrate is vastly more common.
Indeed, the original formula is for the heptahydrate version, but Sigma translated it to the anhydrous.(Knudson 1922)
25 May 2021
Automated sterilizing is worth discussing. A small, autoclavable container that floods and drains a sample in an automated manner.
The choice between parallel or sequential filling/decanting of solutions during sterilization of explants:
The filling and decanting of solutions takes only a short amount of time compared to the soaking time in the case of bleach. For ethanol sterilization, the soak time (~30 seconds) is short enough that the operator can wait. Therefore, automated sterilization should be sequential.
Seeds or explants are placed in appropriately sized autoclavable containers (e.g. Falcon tubes). The appropriate sterilant protocol is selected from the menu and the tube is pushed into the dispensing/evacuating head.
An IR beam-break sensor on the fill head can detect when a tube is loaded for button-free operation.
It would cost me about $0.90 in electricity just to boil 1 gallon of water. It costs $1 to buy distilled water.
Auxin stocks are usually prepared by weighing out 10 mg of auxin into a 200-ml beaker, adding several drops of 1 N NaOH or KOH until the crystals are dissolved (not more than 0.3 ml), rapidly adding 90 ml of double-distilled water, and increasing the volume to 100 ml in a volumetric flask (Huang & Murashige, 1976).
Auxins can also be dissolved in 95% ethanol and diluted to volume; however, ethanol is toxic to plant tissues. The K-salts of auxin are more soluble in water (Posthumus, 1971).
Make IAA stocks fresh weekly; IAA is degraded within a few days by light (Yamakawa et al., 1979; Dunlap & Robacker, 1988) and within several hours to a few days by plant tissues (Epstein & Lavee, 1975). Auxins are thermostable at 110–120°C for up to 1 h (Posthumus, 1971; Yamakawa et al., 1979).
Cytokinin stocks are prepared in a fashion similar to that for auxin stocks, except that 1 N HCl and a few drops of water are used to dissolve the crystals (Huang & Murashige, 1976). Gentle heating is usually required to completely dissolve crystals.
Cytokinin stocks can be stored for several months in the refrigerator.
Cytokinins (kinetin and zeatin) are thermostable; no breakdown products were detected after 1 h at 120°C (Dekhuijzen, 1971); 2iP and BA are stable for 20 min at 100°C.
Most workers add vitamin stock solutions to the medium before autoclav-ing; however, for specific studies on vitamins, they should be filter sterilized (Ten Ham, 1971).(Smith 2013)
1“, 2”, 4" sections of grocery store plastic wrap (polyethylene) were cut. Initially I cracked the lids 1/2 turn on the falcon tubes I used for the single seed germination. I though that this was unnecessarily risky, so I sealed them on day 2. Today I unscrewed the tops again 1/2 turn but then wrapped them in 2 layers of plastic wrap.
A discussion in the Plant-tc listserver mentions the use of Glad-Wrap, which is a polyethylene film with good gas permeability for oxygen, carbon dioxide, and ethylene and with a low permeability for water vapor as compared to Saran-Wrap, a poly-vynilidene copolymer, which has low gas permeability.(Smith 2013)
If this procedure is to continue, perhaps a sock knitting machine could be adapted? Probably over-kill. Besides, for the long-term, filter vents are preferred.
Any type of wrap can create problems in data replication due to variances in the number of layers of wrap and tightness of the wrap. These parameters will make the gas exchange ratios variable.(Smith 2013)
24 May 2021
I solved my seed rinsing problem: A suction wand.
A small heat resistant tube with a sintered filter on one side and a controlled vacuum line on the other. After soaking in sterilant, a seed can be suctioned to the filter and transferred to the next solution or rinsed directly on the filter. The end can be sterilized in a bead sterilizer just like forceps.
If a liquid trap in placed inline, waste solutions can also be suctioned.
I have a HPLC degassing pump that I repaired just waiting to be used for this. It was designed to handle organic solvent vapor, so I definitely can’t hurt the thing.
23 May 2021
Purification
- Potassium sulfate: recrystallize in water; 8ml/g
Monosodium phosphate: recrystallize in water 0.5ml/g
Monopotassium phosphate
Dissolve it in boiling distilled water (2ml/g), keep on a boiling water-bath for several hours, then filter it through paper pulp to remove any turbidity. Cool rapidly with constant stirring, and the crystals are collected on to hardened filter paper, using suction, washed twice with ice-cold water, once with 50% EtOH, and dried at 105C.
- Zinc sulfate: Crystallize from dilute sulfuric acid above 70C for monohydrate.
- Thiamine hydrochloride: Recrystallize from 95% EtOH
- Pyridoxine Hydrochloride: Recrystallize from EtOH+Acetone
- Niacin: Recrystallize from toluene (probably not); vac distill at 160C
- Inositol: Recrystallize from 50% EtOH; Dehydrate at 100C;
- Glycine:
Crystallise glycine from distilled water by dissolving at 90-95C, filtering, cooling to about -5C, and draining the crystals centrifugally. Alternatively, crystallise it from distilled water by addition of MeOH or EtOH (e.g. 50g dissolved in 100ml of warm water, and 400ml of MeOH is added).(Armarego 2017)
- Sodium molybdate
Dehydrates at 100C. Purified by drowning 250g/l molybdate in water solution with absolute ethanol until ~50% EtOH (50g/l molybdate).(Casas and Lagos 2020)
- Ferrous sulfate heptahydrate
Crystallise the sulfate from 0.4M H2SO4, or precipitate it from an aqueous solution with EtOH.
[solubility:] 0.66g/ml(0C), 0.21g/ml(10C), 0.29g/ml(25C), 0.40g/ml(40C) and 0.51g/ml(54C) for the heptahydrate
but it is insoluble in EtOH (Armarego 2017)
21 May 2021
I’ve been reading more about temporary immersion bioreactors. They seem like a wonderful system. Adaptable to a wide variety of growth protocols as well as automation. They are prohibitively expensive, however. So let’s DIY some.
I like the Plantform bioreactor (~$60 each). This might be because they look like they are made from regular tupperware.
2 qt. polycarbonate containers
Triton plastic aka Rubbermaid Brilliance is not autoclavable.
20 May 2021
Started triplicate seeds (except 2 bacopa and 1 morning glory) on full-strength MS media for 15 new species on the 18th. It took about 3 hours in front of the hood. It would have gone faster if the seeds weren’t so difficult to manipulate. Some sort of purpose-built sterilization tube with a built-in filter would be great. Also, a glass bead sterilizer for rapid reuse of forceps.
Tuber melanosporum
Black truffle innoculated seedling of oak or hazelnut would be a worthy addition to my line of products. They are commercially available for ~$30 each.
Traditional inoculation is accomplished via irrigation with spore suspension from ground truffles. This is hit-or-miss and requires a second, visual verification of inoculation. Even then, the genetics are unknown and truffles have a complex life cycle.(Rubini et al. 2012, 2014; Le Tacon et al. 2016)
It has long been assumed that truffles “self” reproduce, that is, a single-individual inoculated tree is capable of producing mature truffles. Research in the last decade has shown that T. melanosporum is, in fact, heterothallic and requires a sexual dimorphic partner to produce mature truffles. This likely also contributes to the paucity of research in in-vitro cultivation. Isolates of T. melanosporum would likely never produce truffles.
In short, there are “male” and “female” T. melanosporum and you don’t get the baby truffles unless you have one of each.
I wonder if morels are the same way… edit: yes (Chai et al. 2017; Liu et al. 2018)
Even if you manage to inoculate two compatible partners on the same tree, given sufficient time, one will dominate. (Rubini et al. 2014)
Host plants can sustain genetically different T. melanosporum strains on their roots, provided that all of the strains share the same mating type.
Alternatively, one could inoculate one tree with a single individual and plant it next to a different tree with a sexually compatible T. melanosporum. An entire plantation of checkerboard mating types could be established.
In theory, plants harboring MAT1-2-1 or MAT1-1-1 strains should be numerically and spatially equally distributed at a site to facilitate reciprocal mating of the fungal partners.
Unfortunately, it is not possible to predict which of the two mating types outcompetes the other on plants inoculated with spores, and screening individual plants to identify the mating types of their symbiotic strain(s) is not feasible due to the cost.
As an alternative to spores, mycelia grown in vitro can be used as inocula. These mycelia can be isolated from mycorrhizas adapted to specific microhabitats and/or from fruiting bodies with a superior quality/size to encourage the propagation of strains that retain useful traits.
However, caution is needed when using plants inoculated by this approach in large-scale cultivation programs because there is a risk of propagating only a few fungal genotypes due to the difficulty of isolating and growing truffle strains in vitro.
It seems that hit-or-miss spore inoculation is still the de-facto standard of plantation establishment.(KnowYourForest 2020)
However, as pointed out earlier, close proximity of male and female gametes of opposite mating types is not sufficient to engage the fertilization process. Unknown factors, which remained to be discovered, are necessary for ascocarp initiation.(Le Tacon et al. 2016)
In vitro culturing might be problematic:
However, after passing through repeated subculturing, aging mycelial cultures tend to lose the ability to establish mycorrhitic associations (Thompson et al., 1993; Roth-Bejerano and Kagan-Zur, unpublished), which severely limits their useful life. What is needed is a way of speeding up mycelial propagation and growth. (Ventura et al. 2006)
The answer: Hairy root cultures!
These results suggest that mycorrhized transformed roots could be the key to an improved system for the production of inoculated seedlings.
Mediterranean Germplasm Database
16 May 2021
Made a foldable work table for the hepa flow hood which is now in the closet.
15 May 2021
Made a box today. Dados are hard with only a circular saw but totally worth it. https://www.hingmy.com/cabinetcalc.php
14 May 2021
13 May 2021
Well the USDA and WFO combination database ran all night. Out of 93,315 usda records, 20,827 did not match WFO records and so were copied into “unknown” table. So around 22%. Not bad considering it was just a straight text compare.
And then I found https://www.gbif.org/.
12 May 2021
The fungiController ran all night without issue. I added a quick sqlite database to record the environmental data.
At some point it would be nice to write a GUI for making new SiLA features. That way you don’t have to know anything about xml or schemas. tutorial
But first I will just write a few by hand (even though I still don’t have a working validator).
Plant information obtained from http://www.worldfloraonline.org/, https://plants.usda.gov/home, https://www.itis.gov/, and others.
csv_to_sqlite makes things easier.
11 May 2021
I got the fungi environmental control system working. Two DS18B20 temperature sensors measure the intake air and wet-bulb temperature. An AM2320 humidity/temp sensor measures the interal environment humidity and temperature. The humidity is controlled by heating the water in the humidifier via relay board and 200 watt immersion heater. Everything is written in python on a RPi Zero W.
Some of the first attempts at attaching leads to the sensors failed for some reason. Maybe I will come back and figure out the problem (I suspect it was just faulty soldering on my part), but for now I just made other ones. I used old USB cabling instead of phone wire. The wire is bigger and stiffer, but that just makes it easier to solder and strip.
Anyway… now I have the fungi controller printing out environment conditions via ssh. I wouldn’t normally include fungi stuff on a plants page, but all of the environmental control systems are going to be in service of plants in the near future.
SiLA2
Note: I am using anaconda as an environment manager. It is recommended that you use venv instead. official instructions
Install the necessary components:
git clone https://gitlab.com/SiLA2/sila_base.git
git clone https://gitlab.com/SiLA2/sila_python.git
cd sila_python/
python sila2install.pyAs a side note: The starting point of SiLA development is an XML document that formally describes the features of your lab equipment. I’ve never worked with XML before, except in passing. The xmlvalidate apm for atom (my default dev environment) is so old that it requires python 2.7 to build and it doesn’t even work.
So I ended up installing notepad-plus-plus. It’s a snap that runs Wine!? Talk about emulator inception. Ugh. Aannnnndddd even that doesn’t work for some reason. I click the “Validate Now” button and nothing happens.
10 May 2021
My right pinky hurts. Too many shift and enter commands.
For some reason the OneWireBus library from Adafruit throws “OneWire has not been implemented” error despite being able to read the temperature directly of both sensors using cat /sys/bus/w1/devices/28-*/w1_slave | grep "t=" command.
So I installed the w1thermsensor library instead.
I also installed the psychrometrics library for easy conversion. The formulas aren’t that complicated and I could write some out to avoid the overhead of the entire library if necessary. https://cals.arizona.edu/azmet/dewpoint.html
For 1-wire sensors, less than 5 meters of cable really don’t matter. I am using 4-conductor telephone wire because it’s basically free. I will get some rj-11 connectors and jacks. They are super cheap.
https://peppe8o.com/raspberry-pi-zero-pinout/
09 May 2021
Incubator still seems to be working. The temperature fluctuates at least +-2C. Gotta work on those environmental control systems.
Since I don’t have a mini USB keyboard, I tried to setup wireless networking and ssh directly on the SD card. I also added static IP info to /etc/dhcpcd.conf.
For some reason that didn’t work, so I hacked together a female USB to male micro USB cable to plug in a keyboard to the pi. Then I reflashed the SD card and booted the right now.
Wifi signal isn’t great. I keep getting get errors. The mylar sheets that line my grow closet and stand between me and the router probably don’t help…
08 May 2021
Started with full strength MS media + MES (commercial mix) + 8g/l agar + 20 g/L table sugar. The media didn’t completely dissolve even after heating to >80C. I poured 30g in 9 1/2 pint jars while swirling to maintain the suspension. The jars are topped with two layers of craft felt and jar lids with 5 1/8" holes (my standard fungi lids). I will pressure cook them at 15psi 121C for 15 minutes to see if everything dissolves appropriately.
I will start with 5 seeds each of Echinopsis langeniformis, E. pachanoi, E. “Serra Blue” X E. pachanoi Monstrose, Lophophora diffusa, and Sceletium tortuosum.
MES concentration 1 g/L (5.1 mM). pH 6.2
It seems that agar melts at +85C, so perhaps I needed to heat the media just a bit more. Yep… that was the problem. Heating to 95C worked.
Weird that I haven’t faced this problem before. Perhaps it’s the pH.
Well the first 5 species are incubating. My sterile technique leaves something to be desired. I forgot to include a surfactant in my bleaching solution. I started steriling the seeds before my sterile water had autoclaved, so I had to improvise some using syringe filters. One of the seeds dropped into the waste beaker during rinsing. I used a bent paperclip to transfer seeds to agar. I definitely put nonsterile items in front of sterile ones.
All in all, I give this a 50% chance of any of the jars surviving without contamination.
Jars are incubating in an aquarium with specially designed filter lid and placed on a seedling heat mat. Full power to the mat results in >30C temperature, so I have it plugged into a variac at 40% power right now.
07 May 2021
L. diffusa:
- full strength MS + 8 g/L agar + 20 g/L sucrose (no PGRs)
- adjust to pH 5.7
- 10 seeds per dish
Planted the wasabi rhizome today. The box it arrived in was severly crushed, but it appears to have survived. The rhizome was planted in a recycled kratky planter filled with hydroponic media (mostly LECA with some river pebbles). Media was PC’d for 15 minutes and the container was washed with bleach prior to use. Twice a day I drench the rhizome with cold tap water until water runs out of the drain hole in the base.
I ordered more tiny fountain pumps. Hopefully I can use them for a more permanent solution. I can’t decide whether I want a DWC or drip system.
06 May 2021
Aaaaannnddd there’s another choice for IoT boards: ESP32. They appear to be a better choice. Integrated wifi. ADCs and DACs. uPython support. The only downside is their lack of display and SD card support unless you want to pay 4x more for the dev board versions which have that support.
You can also buy SD card modules for less than $1. They are basically just breakout boards that connect to the ESP32 via SPI.
https://randomnerdtutorials.com/esp32-microsd-card-arduino
Thinking about culture containers… I like the idea of temporary immersion. Perhaps a coarse charcoal puck at the bottom of the container would work? It could be compressed and bound using a biodegradable glue (perhaps gelatin based). Sort of an in vitro support material like a flood drain hydroponic system.(Hoang et al. 2020) Charcoal would be a natural alternative to vermiculite, perlite, or other non-renewable media. This system would be optimized for the rooting phase. Unless we are talking about a rotating/rocking system…
Ultimately, the customer will receive a wrapped plant suitable for indoor/outdoor planting. If the culture container could double as a shipping container, that would be awesome. The top could be removed after sterile culture and contain more permanent/expensive parts like tubing and connectors.
Temporary immersion would require some sort of binder to keep the support media from surrounding the plant. The RITA culture system uses a built-in filter to separate the liquid from the plants.
There might be a different style container for woody plants: They can’t just float around and bend to root in any direction. There has to be a balance between friability of the substrate and strength.
http://seed.worldveg.org/
05 May 2021
If I can sterilize containers with vacuum hydrogen peroxide, then I can simultaneously sterilize liquids in a continuous, high-temperature short-time process.
https://www.sciencedirect.com/topics/engineering/continuous-sterilisation
Agar might present a problem. At RT, it is solid or undissolved. Either mixing or separate heating might be necessary. Unless we are using liquid media without agar… (???)
Some old design equations. (Deindoerfer and Humphrey 1958)
Unfortunately, this precludes things like grain jars for mushrooms since they can not be sterilized by either of these methods. It might be a possibility in the future, however.
What containers? For short cacti, petri dishes can be used.
Creating research alerts on google:
(“in vitro” OR “in-vitro” OR “micropropagation” OR “tissue culture”) AND [species]
Glass bead sterilizers are $75-$200.
Contact the cactus conservation institute https://www.cactusconservation.org/CCI/Contact.html
Medicinal plant conservation certificate program https://unitedplantsavers.org/medicinal-plant-conservation-certificate-program/
04 May 2021
RPi controlled pressure cooker was looking to be an easy project until I looked at steam solenoid valve prices. They cost more than my pressure cooker. I might be able to get away with some $50 models designed for less demanding steam systems. We aren’t talking nuclear reactor steam vessel here. Just 200kPa at 121C.
I could, of course, manually vent the pressure cooker during the initial warm-up phase. And that is probably what I will do. Complete automation is only reasonable with larger systems.
What about a stepper/servo motor turning a regular valve?
26 Apr 2021
Investigating plant tissue culture. https://www.youtube.com/watch?v=uPuxS1kxdVY
06 Oct 2020
In preparation for my ten day trip, I have secured almost all of the plants in kratky hydroponic containers. Only the succulents and trees remain in soil.
I also turned the lighting down to a minimum. This feature is super nice for keeping the plants from using too much water while I’m away.
25 Sep 2020
Project Created!
I have several medicinal plants that need specific growing conditions. This is a compiled list.
Bibliography
AFREEN, F., S. M. A. ZOBAYED, and T. KOZAI. 2002. “Photoautotrophic Culture of Coffea Arabusta Somatic Embryos: Development of a Bioreactor for Large-Scale Plantlet Conversion from Cotyledonary Embryos.” Annals of Botany 90 (1): 21–29. https://doi.org/10.1093/aob/mcf151.
Ahuja, M. R., ed. 1993. Micropropagation of Woody Plants. Vol. 41. Forestry Sciences. Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-94-015-8116-5.
Armarego, W. L. F. 2017. Purification of Laboratory Chemicals. Eighth edition. Amsterdam: Butterworth-Heinemann is an imprint of Elsevier.
Casas, Jesús, and Josué Lagos. 2020. “Drowning-Out Crystallization of Sodium Molybdate in Aqueous-Ethanol Solutions.” Chemical Industry and Chemical Engineering Quarterly 26 (4): 395–99. https://doi.org/10.2298/CICEQ191029017C.
Chai, Hongmei, Lijiao Chen, Weimin Chen, Qi Zhao, Xiaolei Zhang, Kaimei Su, and Yongchang Zhao. 2017. “Characterization of Mating-Type Idiomorphs Suggests That Morchella Importuna, Mel-20 and M. Sextelata Are Heterothallic.” Mycological Progress 16 (7): 743–52. https://doi.org/10.1007/s11557-017-1309-x.
Deindoerfer, F H, and A E Humphrey. 1958. “Principles in the Design of Continuous Sterilizers’” 7 (December): 7.
Faber, Richard James. 2019. “Vegetative Growth and Alkaloid Concentration of Sceletium Tortuosum (L.) N.E. Br. in Response to Different Soilless Growing Media and Fertigation Regimes in Hydroponics.” Thesis, Cape Peninsula University of Technology. http://etd.cput.ac.za/handle/20.500.11838/3108.
Ghasheem, Nazar AL, Adrian George Peticil, and Oana Venat. 2018. “In Vitro Effect of Various Sterilization Techniques on Peach (Prunux Persica (L.)) Explants.” Horticulture, B, 62: 8.
Gupta, S. Dutta, and Yasuomi Ibaraki, eds. 2006. Plant Tissue Culture Engineering. Focus on Biotechnology, v. 6. Dordrecht: Springer.
H, Moradkhani, Sargsyan E, Bibak H, Naseri B, Sadat-Hosseini M, Fayazi-Barjin A, and Meftahizade H. 2010. “Melissa Officinalis L., a Valuable Medicine Plant: A Review.” Journal of Medicinal Plants Research 4 (25): 2753–9. https://doi.org/10.5897/JMPR.9000881.
Hoagland, D. R., and D. I. Arnon. 1950. “The Water-Culture Method for Growing Plants Without Soil.” Circular. California Agricultural Experiment Station 347 (2nd edit). https://www.cabdirect.org/cabdirect/abstract/19500302257.
Hoang, Nhung Ngoc, Yoshiaki Kitaya, Toshio Shibuya, and Ryosuke Endo. 2020. “Effects of Supporting Materials in in Vitro Acclimatization Stage on Ex Vitro Growth of Wasabi Plants.” Scientia Horticulturae 261 (February): 109042. https://doi.org/10.1016/j.scienta.2019.109042.
KnowYourForest. 2020. “Tree School Online: Commercial Truffle Cultivation in Western Oregon.” https://www.youtube.com/watch?v=kVys_mrpy-Y.
Knudson, Lewis. 1922. “Nonsymbiotic Germination of Orchid Seeds.” Botanical Gazette 73 (1): 1–25. https://doi.org/10.1086/332956.
Le Tacon, François, Andrea Rubini, Claude Murat, Claudia Riccioni, Christophe Robin, Beatrice Belfiori, Bernd Zeller, et al. 2016. “Certainties and Uncertainties About the Life Cycle of the Périgord Black Truffle (Tuber Melanosporum Vittad.).” Annals of Forest Science 73 (1): 105–17. https://doi.org/10.1007/s13595-015-0461-1.
Liu, Wei, LianFu Chen, YingLi Cai, QianQian Zhang, and YinBing Bian. 2018. “Opposite Polarity Monospore Genome de Novo Sequencing and Comparative Analysis Reveal the Possible Heterothallic Life Cycle of Morchella Importuna.” International Journal of Molecular Sciences 19 (9). https://doi.org/10.3390/ijms19092525.
Mohamad Zuldin, Nor Nahazima, Ikram Md Said, Normah Mohd Noor, Zamri Zainal, Chew Jin Kiat, and Ismanizan Ismail. 2013. “Induction and Analysis of the Alkaloid Mitragynine Content of a Mitragyna Speciosa Suspension Culture System Upon Elicitation and Precursor Feeding.” The Scientific World Journal 2013 (August): e209434. https://doi.org/10.1155/2013/209434.
Moyano, Elisabeth, S. Mangas, L. Hernandez-Vasquez, and M. Bonfill. 2009. “Centella Asiatica (L) Urban: An Updated Approach.” Plant Secondary Terpenoids, 55–74. https://www.academia.edu/26895227/Centella_asiatica_L_Urban_An_updated_approach.
Nookabkaew, Sumontha, Nuchanart Rangkadilok, Norratouch Prachoom, and Jutamaad Satayavivad. 2016. “Concentrations of Trace Elements in Organic Fertilizers and Animal Manures and Feeds and Cadmium Contamination in Herbal Tea (Gynostemma Pentaphyllum Makino).” Journal of Agricultural and Food Chemistry 64 (16): 3119–26. https://doi.org/10.1021/acs.jafc.5b06160.
Nuwansi, K. K. T., A. K. Verma, G. Rathore, Chandra Prakash, M. H. Chandrakant, and G. P. W. A. Prabhath. 2019. “Utilization of Phytoremediated Aquaculture Wastewater for Production of Koi Carp (Cyprinus Carpio Var. Koi) and Gotukola (Centella Asiatica) in an Aquaponics.” Aquaculture 507 (May): 361–69. https://doi.org/10.1016/j.aquaculture.2019.04.053.
Patel, Ashok Kumar, Deepika Lodha, and Narpat S. Shekhawat. 2020. “An Improved Micropropagation Protocol for the Ex Situ Conservation of Mitragyna Parvifolia (Roxb.) Korth. (Rubiaceae): An Endangered Tree of Pharmaceutical Importance.” In Vitro Cellular & Developmental Biology - Plant 56 (6): 817–26. https://doi.org/10.1007/s11627-020-10089-6.
Prasad, Archana, Archana Mathur, Manju Singh, Madan M. Gupta, Girish C. Uniyal, Raj K. Lal, and Ajay K. Mathur. 2012. “Growth and Asiaticoside Production in Multiple Shoot Cultures of a Medicinal Herb, Centella Asiatica (L.) Urban, Under the Influence of Nutrient Manipulations.” Journal of Natural Medicines 66 (2): 383–87. https://doi.org/10.1007/s11418-011-0588-9.
Prasad, Archana, V. S. Pragadheesh, Archana Mathur, N. K. Srivastava, Manju Singh, and A. K. Mathur. 2012. “Growth and Centelloside Production in Hydroponically Established Medicinal Plant-Centella Asiatica (L.).” Industrial Crops and Products 35 (1): 309–12. https://doi.org/10.1016/j.indcrop.2011.06.020.
Radácsi, Péter, Krisztina Szabó, Dóra Szabó, Eszter Trócsányi, and Éva Németh-Zámbori. 2016. “Effect of Water Deficit on Yield and Quality of Lemon Balm (Melissa Officinalis L.).” Zemdirbyste-Agriculture 103 (4): 385–90. https://doi.org/10.13080/z-a.2016.103.049.
Rubini, Andrea, Beatrice Belfiori, Claudia Riccioni, and Francesco Paolocci. 2012. “Genomics of Tuber Melanosporum: New Knowledge Concerning Reproductive Biology, Symbiosis, and Aroma Production.” In Edible Ectomycorrhizal Mushrooms, edited by Alessandra Zambonelli and Gregory M Bonito, 34:57–72. Berlin, Heidelberg: Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-642-33823-6_4.
Rubini, Andrea, Claudia Riccioni, Beatrice Belfiori, and Francesco Paolocci. 2014. “Impact of the Competition Between Mating Types on the Cultivation of Tuber Melanosporum: Romeo and Juliet and the Matter of Space and Time.” Mycorrhiza 24 (1): 19–27. https://doi.org/10.1007/s00572-013-0551-6.
Smith, M. T., N. R. Crouch, N. Gericke, and M. Hirst. 1996. “Psychoactive Constituents of the Genus Sceletium N.E.Br. and Other Mesembryanthemaceae: A Review.” Journal of Ethnopharmacology 50 (3): 119–30. https://doi.org/10.1016/0378-8741(95)01342-3.
Smith, Roberta H. 2013. Plant Tissue Culture: Techniques and Experiments. Third edition. Amsterdam: Academic Press.
Vacin, Emil F., and F. W. Went. 1949. “Some pH Changes in Nutrient Solutions.” Botanical Gazette 110 (4): 605–13. https://doi.org/10.1086/335561.
Ventura, Yvonne, David Mills, Varda Kagan-Zur, Nurit Roth-Bejerano, and Amnon Bustan. 2006. “Mycorrhized Ri-Transformed Roots Facilitate in Vitro Inoculation of Cistus Incanus with Tuber Melanosporum.” Plant Cell, Tissue and Organ Culture 85 (1): 53–61. https://doi.org/10.1007/s11240-005-9048-0.
Zhang, Mengzi, Abhisheak Sharma, Francisco León, Bonnie Avery, Roger Kjelgren, Christopher R. McCurdy, and Brian J. Pearson. 2020. “Effects of Nutrient Fertility on Growth and Alkaloidal Content in Mitragyna Speciosa (Kratom).” Frontiers in Plant Science 11. https://doi.org/10.3389/fpls.2020.597696.